| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
 O
O is an exotic stable compound
is an exotic stable compound For the Mg-rich part of Mg-O phase diagram, USPEX shows completely unexpected results. First of all, elemental Mg is predicted to undergo several phase transitions induced by pressure: hcp - bcc - fcc - sh. At ambient conditions, Mg adopts the hcp structure, while bcc-Mg is stable from 50 GPa to 456 GPa, followed by the transition to fcc and simple hexagonal phase at 456 and 756 GPa, respectively. These results are in excellent agreement with previous studies 190; 191; 192. Unexpectedly, Mg-rich oxides, such as Mg O and Mg
O and Mg O
O begin to show very competitive enthalpy of formation at pressures above 100 GPa. However, they are still not stable against decomposition into Mg and MgO, and their crystal structures could be thought of as a combination of blocks of Mg and B1-MgO. This situation qualitatively changes at 500 GPa, where we find that Mg
begin to show very competitive enthalpy of formation at pressures above 100 GPa. However, they are still not stable against decomposition into Mg and MgO, and their crystal structures could be thought of as a combination of blocks of Mg and B1-MgO. This situation qualitatively changes at 500 GPa, where we find that Mg O
O becomes thermodynamically stable. This new stable (
becomes thermodynamically stable. This new stable ( -Mg
-Mg O
O ) phase has a very unusual tetragonal structure with the space group P4/mbm.
) phase has a very unusual tetragonal structure with the space group P4/mbm.
![\includegraphics[scale=0.6]{chapter6/pdf/fig3-Mg3O2.png}](images/img-0285.png)
 -Mg
-Mg O
O at 500 GPa, space group P4/mbm, a=4.508 , c=2.367 , Mg1(0.3494, 0.1506, 0.5); Mg2(0, 0, 0); O(0.8468, 0.6532, 0). (b) highlight of the 1D-column of body-centered Mg-cubes.
at 500 GPa, space group P4/mbm, a=4.508 , c=2.367 , Mg1(0.3494, 0.1506, 0.5); Mg2(0, 0, 0); O(0.8468, 0.6532, 0). (b) highlight of the 1D-column of body-centered Mg-cubes.![\includegraphics[scale=0.6]{chapter6/pdf/fig4-Mg-MgO.png}](images/img-0286.png)
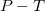 stability field and decomposition conditions of
stability field and decomposition conditions of  -Mg
-Mg O
O .
.This crystal structure can be viewed as a packing of O atoms and 1D-columns of almost perfect body-centered Mg-cubes. As shown in Fig. 6.3, there are two types of Mg atoms in the unit cell, Mg1 and Mg2. Here, Mg2 atoms form the cubes, merged into vertical columns and filled by Mg1 atoms. Within the cubic columns, one can notice empty (Mg1) (Mg2)
(Mg2) clusters with the shape of flattened octahedra, with Mg-Mg distances ranging from 2.08 (Mg1-Mg2) to 2.43 (Mg2-Mg2). The coordination environments are quite different: each Mg1 is bonded to two Mg1 atoms and eight Mg2 atoms, while each Mg2 atoms is bonded to six O atoms (trigonal prismatic coordination). Oxygen atoms in t-Mg
clusters with the shape of flattened octahedra, with Mg-Mg distances ranging from 2.08 (Mg1-Mg2) to 2.43 (Mg2-Mg2). The coordination environments are quite different: each Mg1 is bonded to two Mg1 atoms and eight Mg2 atoms, while each Mg2 atoms is bonded to six O atoms (trigonal prismatic coordination). Oxygen atoms in t-Mg O
O are coordinated by eight Mg2 atoms.
are coordinated by eight Mg2 atoms.