| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
What are the implications of these two compounds for planetary sciences? High pressures, required for their stability, are within the range corresponding to deep planetary interiors. In planetary interiors, reducing conditions dominate, related to the excess of metallic iron. However, given the diversity of planetary bodies it is not impossible to imagine that on some planets strongly oxidized environments can be present at depths corresponding to the pressure of 116 GPa (in the Earth this corresponds to depths below 2600 km). At the more usual reducing conditions of planetary interiors, Mg O
O could exist at pressures above 500 GPa in deep interiors of giant planets. There, it can coexist in equilibrium with Fe (but probably not with FeO, according to our DFT and DFT+U calculations of the reaction Fe + 3MgO
could exist at pressures above 500 GPa in deep interiors of giant planets. There, it can coexist in equilibrium with Fe (but probably not with FeO, according to our DFT and DFT+U calculations of the reaction Fe + 3MgO 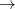 FeO + Mg
FeO + Mg O
O ). According to our calculations (Fig. 6.4), Mg
). According to our calculations (Fig. 6.4), Mg O
O can only be stable at temperatures below 1800 K, which is too cold for deep interiors of giant planets; however, impurities and entropy effects stemming from defects and disorder might extend its stability field into planetary temperatures. Exotic compounds MgO
can only be stable at temperatures below 1800 K, which is too cold for deep interiors of giant planets; however, impurities and entropy effects stemming from defects and disorder might extend its stability field into planetary temperatures. Exotic compounds MgO and Mg
and Mg O
O , in addition to their general chemical interest, might be important planet-forming minerals in deep interiors of some planets.
, in addition to their general chemical interest, might be important planet-forming minerals in deep interiors of some planets.