| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Glycine, with the formula NH CH
CH COOH, is the smallest of 20 aminoacids commonly found in proteins. Aminoacids are important in nutrition and widely used in the pharmaceutical industry.
COOH, is the smallest of 20 aminoacids commonly found in proteins. Aminoacids are important in nutrition and widely used in the pharmaceutical industry.
The polymorphism of glycine is intensively investigated 112; 113; 114; 115; 116; 117; 118. Glycine is known to crystallize in four polymorphs with space groups P2 /c, P2
/c, P2 , P3
, P3 and P2
and P2 /c which are labeled
/c which are labeled  ,
, 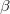 ,
, 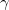 and
and  , respectively 112. The glycine molecule is pictured in Fig. 3.10a. The
, respectively 112. The glycine molecule is pictured in Fig. 3.10a. The  ,
, 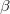 , and
, and 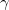 phases are found at ambient pressure, with
phases are found at ambient pressure, with  and
and 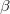 phases being metastable with respect to the
phases being metastable with respect to the 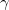 phase.
phase.  glycine has recently been found to form under pressure 114. In the gas phase, glycine is in a nonionic form, while in all four of the crystal structures glycine is zwitterionic (as shown in Fig. 3.10a). In this form, an -NH
glycine has recently been found to form under pressure 114. In the gas phase, glycine is in a nonionic form, while in all four of the crystal structures glycine is zwitterionic (as shown in Fig. 3.10a). In this form, an -NH
 group on one ion electrostatically interacts with a -COO
group on one ion electrostatically interacts with a -COO group on a neighboring ion. Although zwitterionization causes an increase in energy with respect to the gas-phase molecule, it is thought that the zwitterionic crystals are stabilized by the increase in number of hydrogen bonds that can be formed in comparison to the number that would be formed in the nonionic case.
group on a neighboring ion. Although zwitterionization causes an increase in energy with respect to the gas-phase molecule, it is thought that the zwitterionic crystals are stabilized by the increase in number of hydrogen bonds that can be formed in comparison to the number that would be formed in the nonionic case.
Since the glycine zwitterion only has the point symmetry C (i.e. no symmetry), structure prediction of glycine is even more challenging compared with benzene. We performed variable cell prediction at 1 GPa with 2 - 4 molecules per cell. Without any experimental information, we found
(i.e. no symmetry), structure prediction of glycine is even more challenging compared with benzene. We performed variable cell prediction at 1 GPa with 2 - 4 molecules per cell. Without any experimental information, we found 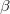 -glycine (Fig. 3.10c) as the metastable structure with Z = 2; and
-glycine (Fig. 3.10c) as the metastable structure with Z = 2; and 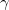 -glycine (Fig. 3.10d) as the best structure with Z = 3. We also found
-glycine (Fig. 3.10d) as the best structure with Z = 3. We also found  -glycine as the metastable form in the calculation with Z = 4 (Fig. 3.10b) at 2.0 GPa. Relative stability is, however, in poor agreement with experimental observations. GGA+D results show that
-glycine as the metastable form in the calculation with Z = 4 (Fig. 3.10b) at 2.0 GPa. Relative stability is, however, in poor agreement with experimental observations. GGA+D results show that  glycine possesses the lowest enthalpy, while
glycine possesses the lowest enthalpy, while 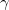 and
and 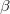 phase are 20 meV/molecule and 30 meV/molecule higher, respectively. However, the experimental results demonstrated the relative thermodynamic stability to be
phase are 20 meV/molecule and 30 meV/molecule higher, respectively. However, the experimental results demonstrated the relative thermodynamic stability to be 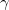
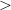

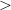
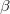 . This shows the need for better ways of computing intermolecular interaction energies.
. This shows the need for better ways of computing intermolecular interaction energies.
![\includegraphics[scale=0.4]{chapter3/pdf/Fig9.png}](images/img-0211.png)
 -glycine at 2 GPa(Z=4, a=5.390 , b=5.911 , c=10.189 ,
-glycine at 2 GPa(Z=4, a=5.390 , b=5.911 , c=10.189 , 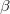 =113.2
=113.2  ); c)
); c) 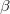 -glycine at 0.4 GPa(Z=2, a=5.372 , b=6.180 , c=5.143 ,
-glycine at 0.4 GPa(Z=2, a=5.372 , b=6.180 , c=5.143 , 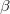 =111.9
=111.9  ); d)
); d) 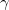 -glycine at 1 GPa(Z=3, a=b=7.070 , c=5.490 ).
-glycine at 1 GPa(Z=3, a=b=7.070 , c=5.490 ).