| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Benzene is the simplest aromatic compound, and it has a purely planar molecule, the packing of which is stabilized by 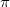 -
-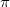 interactions. The crystal structure of benzene is one of the most basic and most actively investigated structures. The first proposed phase diagram was very complex and contained six solid phases 107. However, recent experimental studies simplified it 108; 109. At normal conditions, benzene crystallizes in the orthorhombic phase I (Pbca). A monoclinic phase II (P2
interactions. The crystal structure of benzene is one of the most basic and most actively investigated structures. The first proposed phase diagram was very complex and contained six solid phases 107. However, recent experimental studies simplified it 108; 109. At normal conditions, benzene crystallizes in the orthorhombic phase I (Pbca). A monoclinic phase II (P2 /c), with two molecules per unit cell was identified above 1.75 GPa. Phase II is stable up to the onset of a chemical reactions (at 41 GPa and 298 K).
/c), with two molecules per unit cell was identified above 1.75 GPa. Phase II is stable up to the onset of a chemical reactions (at 41 GPa and 298 K).
In our simulation we started with the same empirical potential 110 as used in a recent metadynamics study 77, and we reproduced the multiple phases of benzene on the old phase diagram. This potential was calibrated at normal conditions and may fail at high pressure, and predicted many stable phases to be stable at different pressures (this is consistent with the old phase diagram, but most of these phases should be metastable according to the new experiment). To remedy this issue, we repeated our structure prediction runs at the level of DFT+D. We performed the calculation at 0, 5, 10, 25 GPa with Z=4. In our simulation, the experimentally observed orthorhombic phase (Pbca) was identified as the most stable phase at 0 GPa, and then it transforms to P4 2
2 2 phase at 4 GPa. We also found the monoclinic phase (P2
2 phase at 4 GPa. We also found the monoclinic phase (P2 /c) (Fig. 3.9) as the ground state above 10 GPa. Our DFT+D results give fewer stable phases, in agreement with the new phase diagram - the only difference is in the P4
/c) (Fig. 3.9) as the ground state above 10 GPa. Our DFT+D results give fewer stable phases, in agreement with the new phase diagram - the only difference is in the P4 2
2 2 phase. This phase is experimentally known, and according to the latest experimental results 108; 109 is metastable. Previous DFT calculation 111 suggested this phase to be stable at pressures 4-7 GPa, which is consistent with our results. Thermodynamic stability of the P4
2 phase. This phase is experimentally known, and according to the latest experimental results 108; 109 is metastable. Previous DFT calculation 111 suggested this phase to be stable at pressures 4-7 GPa, which is consistent with our results. Thermodynamic stability of the P4 2
2 2 phase needs to be reappraised experimentally.
2 phase needs to be reappraised experimentally.
![\includegraphics[scale=0.4]{chapter3/pdf/Fig8.png}](images/img-0206.png)
 2
2 2, Z=4); (c) monoclinic phase (P2
2, Z=4); (c) monoclinic phase (P2 /c, Z=2).
/c, Z=2).