| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
The CO molecule has a special significance because it is very abundant in nature and is a model system involving
molecule has a special significance because it is very abundant in nature and is a model system involving 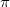 bonding and sp-hybridization of carbon atoms. Similar to methane, carbon dioxide is a vdW crystal with strong (weak) intra-molecular (inter-molecular) interactions at low pressures 100. At room temperature and 1.5 GPa, CO
bonding and sp-hybridization of carbon atoms. Similar to methane, carbon dioxide is a vdW crystal with strong (weak) intra-molecular (inter-molecular) interactions at low pressures 100. At room temperature and 1.5 GPa, CO crystallizes as dry ice, with a cubic Pa3 structure. At pressures between 12 and 20 GPa, CO
crystallizes as dry ice, with a cubic Pa3 structure. At pressures between 12 and 20 GPa, CO -I transforms to another molecular solid CO
-I transforms to another molecular solid CO -III 101; 102; 103. The structure of phase III has been determined to be orthorhombic (Cmca) from X-ray diffraction experiments up to 12 GPa. It is known that above 20 GPa a non-molecular phase (called phase V) with tetrahedrally coordinated carbon atoms becomes stable 100.
-III 101; 102; 103. The structure of phase III has been determined to be orthorhombic (Cmca) from X-ray diffraction experiments up to 12 GPa. It is known that above 20 GPa a non-molecular phase (called phase V) with tetrahedrally coordinated carbon atoms becomes stable 100.
In the previous prediction 104, unconstrained USPEX calculations succeeded in finding the correct CO structures in a wide pressure range. By applying molecular constraint, we have more easily found the Cmca phase, just in 4 generations or
structures in a wide pressure range. By applying molecular constraint, we have more easily found the Cmca phase, just in 4 generations or  140 structural relaxations (Fig. 3.8). Cmca phase remains the most stable structure made of discrete CO
140 structural relaxations (Fig. 3.8). Cmca phase remains the most stable structure made of discrete CO molecules at least up to 80 GPa. Both experiment 105 and theory 104; 106 show that CO
molecules at least up to 80 GPa. Both experiment 105 and theory 104; 106 show that CO polymerizes above 20 GPa while the molecular form (Cmca phase) exists as a metastable form at low temperatures and higher pressures. This examples shows how imposing constraints gives the most stable molecular form, while unconstrained search finds the global minimum (which for CO
polymerizes above 20 GPa while the molecular form (Cmca phase) exists as a metastable form at low temperatures and higher pressures. This examples shows how imposing constraints gives the most stable molecular form, while unconstrained search finds the global minimum (which for CO is non-molecular above 20 GPa). Both cases correspond to situations that are experimentally achievable, and thus important.
is non-molecular above 20 GPa). Both cases correspond to situations that are experimentally achievable, and thus important.
![\includegraphics[scale=0.4]{chapter3/pdf/Fig7.png}](images/img-0205.png)
 III (space group: Cmca, Z=4).
III (space group: Cmca, Z=4).