| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
In the previous chapter, we discuss the work of variable stoichiometries study on Xe-O system, where we need to manually try different chemical compositions to construct the convex hull. we use the new function of USPEX which could allow one to optimize the convex. Therefore, one does not need to specify the chemical composition and simply running one single prediction could in principle yield all thermodynamically stable stoichiometries and the corresponding crystal structures. In this chapter, we employ the function to study another system, magnesium oxide.
Using ab initio evolutionary simulations, we explore all the entire range of possible stoichiometries for Mg-O system at pressures up to 850 GPa. In addition to MgO, our calculations find that two extraordinary compounds MgO and Mg
and Mg O
O become thermodynamically stable at 116 GPa and 500 GPa, respectively. Detailed chemical bonding analysis shows large charge transfer in all magnesium oxides. MgO
become thermodynamically stable at 116 GPa and 500 GPa, respectively. Detailed chemical bonding analysis shows large charge transfer in all magnesium oxides. MgO contains peroxide ions [O-O]
contains peroxide ions [O-O]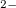 , while non-nuclear electron density maxima play the role of anions in the electride compound Mg
, while non-nuclear electron density maxima play the role of anions in the electride compound Mg O
O . The latter compound is calculated to have a much narrower band gap compared to MgO and MgO
. The latter compound is calculated to have a much narrower band gap compared to MgO and MgO . We discuss conditions at which MgO
. We discuss conditions at which MgO and Mg
and Mg O
O could exist in planetary conditions.
could exist in planetary conditions.