| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
In summary, we performed a systematic search for possible stoichiometries in the Mg-O system at pressures up to 850 GPa. Other than the well-known compound, MgO, we found that two more stoichiometries (MgO and Mg
and Mg O
O ) become thermodynamically stable at pressures above 116 GPa and 500 GPa, respectively. Our analysis reveals that bonding in both insulating
) become thermodynamically stable at pressures above 116 GPa and 500 GPa, respectively. Our analysis reveals that bonding in both insulating  -MgO
-MgO and semiconduting
and semiconduting  -Mg
-Mg O
O exhibits significantly ionic character. MgO
exhibits significantly ionic character. MgO is stabilized by the formation of the peroxide [O-O]
is stabilized by the formation of the peroxide [O-O]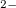 anion, while the strong electron localization in Mg octahedron plays the role of an additional anion and makes the anion-deficient compound Mg
anion, while the strong electron localization in Mg octahedron plays the role of an additional anion and makes the anion-deficient compound Mg O
O stable. These two compounds might exist in interiors of other planets, and are thus potentially important for our fundamental understanding of the universe - in addition to presenting new striking chemical phenomena.
stable. These two compounds might exist in interiors of other planets, and are thus potentially important for our fundamental understanding of the universe - in addition to presenting new striking chemical phenomena.