| Crystal Structure Prediction and its Application in Earth and Materials Sciences |

It is well known that monovalent (H-Cs) and divalent (Be-Ba and Zn-Hg) elements are able to form not only normal oxides, but also peroxides and even superoxides 186 (for instance, BaO has been well studied at both ambient and high pressure 187; 188). Our structure prediction calculations identified the presence magnesium peroxide with Pa3 symmetry and 12 atoms in the unit cell at ambient pressure, which is in good agreement with experimental results 189. In this cubic phase, Mg is octahedrally coordinated by oxygen atoms (which form O
has been well studied at both ambient and high pressure 187; 188). Our structure prediction calculations identified the presence magnesium peroxide with Pa3 symmetry and 12 atoms in the unit cell at ambient pressure, which is in good agreement with experimental results 189. In this cubic phase, Mg is octahedrally coordinated by oxygen atoms (which form O dumbbells), see Fig. 6.2a. However, Pa3 MgO
dumbbells), see Fig. 6.2a. However, Pa3 MgO (
( -MgO
-MgO from now on) is calculated to have a positive formation enthalpy from Mg and O
from now on) is calculated to have a positive formation enthalpy from Mg and O , and is therefore metastable. The calculation shows that on increasing pressure,
, and is therefore metastable. The calculation shows that on increasing pressure,  -MgO
-MgO transforms into a tetragonal form with the space group I4/mcm. In the
transforms into a tetragonal form with the space group I4/mcm. In the  -MgO
-MgO phase (Fig. 6.2b), Mg is 8-coordinate. Here we see the same trend of change from 6-fold to 8-fold coordination as in the predicted B1-B2 transition in MgO. but in MgO
phase (Fig. 6.2b), Mg is 8-coordinate. Here we see the same trend of change from 6-fold to 8-fold coordination as in the predicted B1-B2 transition in MgO. but in MgO it happens at mere 53 GPa, compared to 490 GPa for MgO. Most remarkably, above 116 GPa the
it happens at mere 53 GPa, compared to 490 GPa for MgO. Most remarkably, above 116 GPa the  -MgO
-MgO structure has a negative enthalpy of formation from MgO and O
structure has a negative enthalpy of formation from MgO and O , indicating that
, indicating that  -MgO
-MgO becomes thermodynamically stable. Furthermore, its stability is greatly enhanced by pressure and its enthalpy of formation becomes impressively negative, -0.43 eV/atom, at 500 GPa! We also examined the effect of temperature on its stability by performing quasiharmonic free energy calculations. Thermal effects tend to decrease the relative stability of MgO
becomes thermodynamically stable. Furthermore, its stability is greatly enhanced by pressure and its enthalpy of formation becomes impressively negative, -0.43 eV/atom, at 500 GPa! We also examined the effect of temperature on its stability by performing quasiharmonic free energy calculations. Thermal effects tend to decrease the relative stability of MgO by 0.008 meV/(atom
by 0.008 meV/(atom K, which is clearly insufficient to change the sign of the formation free energy (
K, which is clearly insufficient to change the sign of the formation free energy (
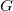 ), and MgO
), and MgO remains stable at high temperatures.
remains stable at high temperatures.
![\includegraphics[scale=1.0]{chapter6/pdf/fig1-MgO-O.png}](images/img-0283.png)
 as a function of pressure. For oxygen, we used the earlier predicted structures in Chapter 6. For MgO, B1 and B2 phases were considered.
as a function of pressure. For oxygen, we used the earlier predicted structures in Chapter 6. For MgO, B1 and B2 phases were considered.  -MgO
-MgO was considered at 50 GPa, while
was considered at 50 GPa, while  -MgO
-MgO was used at pressures higher than 50 GPa.
was used at pressures higher than 50 GPa.![\includegraphics[scale=0.6]{chapter6/pdf/fig2-MgO2.png}](images/img-0284.png)
 -MgO
-MgO phase at 50 GPa, space group Pa3, a=4.524 , Mg(0, 0, 0), O(0.4074, 0.4074, 0.4074); (b)
phase at 50 GPa, space group Pa3, a=4.524 , Mg(0, 0, 0), O(0.4074, 0.4074, 0.4074); (b) -MgO
-MgO at 500 GPa, space group I4/mcm, a=3.377 , c=3.985 , Mg(0, 0, 0.75), O(0.1260, 0.3740, 0.5). The green polyhedra are drawn to show the coordination environment of Mg atoms (6-fold in
at 500 GPa, space group I4/mcm, a=3.377 , c=3.985 , Mg(0, 0, 0.75), O(0.1260, 0.3740, 0.5). The green polyhedra are drawn to show the coordination environment of Mg atoms (6-fold in  -MgO
-MgO , and 8-fold in
, and 8-fold in  -MgO
-MgO ).
).