| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
To summarize, we have predicted stability of xenon oxides at high pressure, which can be readily tested experimentally. On increasing pressure, increasingly high oxidation states of Xe will appear - first, XeO (above 83 GPa), then XeO (above 102 GPa), then XeO
(above 102 GPa), then XeO (above 114 GPa). Present results clearly show that Xe loses its chemical inertness under pressure and that charge transfer plays an essential role in chemical bonding of Xe compounds and their stability is largely determined by electronegativity differences. Furthermore, pressure stabilizes increasing oxidation states of Xe atoms (Xe
(above 114 GPa). Present results clearly show that Xe loses its chemical inertness under pressure and that charge transfer plays an essential role in chemical bonding of Xe compounds and their stability is largely determined by electronegativity differences. Furthermore, pressure stabilizes increasing oxidation states of Xe atoms (Xe
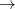 Xe
Xe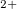
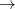 Xe
Xe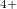
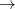 Xe
Xe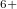 ) and enhances charge transfer from Xe to O atoms. We find that xenon silicates are not stable at pressures of the Earth’s mantle (
) and enhances charge transfer from Xe to O atoms. We find that xenon silicates are not stable at pressures of the Earth’s mantle (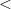 136 GPa) and that xenon oxides, although stable against decomposition into the elements, will be reduced to free Xe in the strongly reducing lower mantle and thus also cannot exist in the Earth’s mantle. While the formation of stable Xe oxides or silicates is not possible at conditions of the Earth’s mantle, the formation of strong Xe-O bonds under pressure, clearly seen in our results, implies that Xe may still be retained at point or line defects, or grain boundaries of mantle minerals. Xenon could also be stored in perovskite/post-perovskite stacking faults171. The facile chemical bonding between Xe and O atoms demonstrated here and the preference of Xe atoms to terminate the silicate perovskite layers, observed in our simulations (xenon silicate in Supplementary Materials), both suggest this possibility. Indeed, the effect of trapping of trace elements by lattice defects is well known 172
136 GPa) and that xenon oxides, although stable against decomposition into the elements, will be reduced to free Xe in the strongly reducing lower mantle and thus also cannot exist in the Earth’s mantle. While the formation of stable Xe oxides or silicates is not possible at conditions of the Earth’s mantle, the formation of strong Xe-O bonds under pressure, clearly seen in our results, implies that Xe may still be retained at point or line defects, or grain boundaries of mantle minerals. Xenon could also be stored in perovskite/post-perovskite stacking faults171. The facile chemical bonding between Xe and O atoms demonstrated here and the preference of Xe atoms to terminate the silicate perovskite layers, observed in our simulations (xenon silicate in Supplementary Materials), both suggest this possibility. Indeed, the effect of trapping of trace elements by lattice defects is well known 172