| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
We did a preliminary test at 20 GPa starting from the graphite-2H structure, and successfully found diamond as the ground state, and M-carbon and bct-C as metastable states. In the calculation we set
as metastable states. In the calculation we set 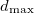 =2.5 ,
=2.5 , 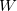 =4000 kbar
=4000 kbar
 and
and 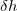 =0.6 .
=0.6 .
![\includegraphics[scale=0.7]{chapter8/pdf/Fig6.png}](images/img-0330.png)
Fig. 8.6 shows the enthalpy evolution. Graphene layers (Fig. 8.7a), persisted until the 15th generation. Then, upon sufficient cell deformation, the layers began to buckle, and the planar structure transformed into 3D-networks of sp -hybridized carbon atoms. Lonsdaleite with 6-membered rings (Fig. 8.7b) appeared as the best structure in the 16th generation. We observed in the same generation the bct-C
-hybridized carbon atoms. Lonsdaleite with 6-membered rings (Fig. 8.7b) appeared as the best structure in the 16th generation. We observed in the same generation the bct-C structure with 4+8 membered rings (Fig. 8.7c), and M/W-carbon structures containing 5+7 membered rings (Fig. 8.7e,f), appeared shortly after. Lonsdaleite survived for a few generations until it transformed into a hybrid structure made of alternating layers of M-carbon and diamond (Fig. 8.7g, we refer to it as M+D type), followed by the transition to another hybrid structure made of bct-C
structure with 4+8 membered rings (Fig. 8.7c), and M/W-carbon structures containing 5+7 membered rings (Fig. 8.7e,f), appeared shortly after. Lonsdaleite survived for a few generations until it transformed into a hybrid structure made of alternating layers of M-carbon and diamond (Fig. 8.7g, we refer to it as M+D type), followed by the transition to another hybrid structure made of bct-C and diamond (Fig. 8.7d, similarly, we refer to it as B+D type). Diamond was dominant in the following generations. At the 51st generation, the system reverted to graphite.
and diamond (Fig. 8.7d, similarly, we refer to it as B+D type). Diamond was dominant in the following generations. At the 51st generation, the system reverted to graphite.
![\includegraphics[scale=0.5]{chapter8/pdf/Fig7.png}](images/img-0331.png)
 2
2  2 supercell of the calculation model); (b) lonsdaleite; (c) bct-C
2 supercell of the calculation model); (b) lonsdaleite; (c) bct-C with 4+8 membered rings; (d) Z-carbon (belonging to B+D type); (e) M-carbon with 5+7 membered rings; (f) W-carbon with 5+7 membered rings; (g) M+D type carbon. Polygons are highlighted by different colors (quadrangle: turquoise; pentagon: green; hexagon: blue).
with 4+8 membered rings; (d) Z-carbon (belonging to B+D type); (e) M-carbon with 5+7 membered rings; (f) W-carbon with 5+7 membered rings; (g) M+D type carbon. Polygons are highlighted by different colors (quadrangle: turquoise; pentagon: green; hexagon: blue).The power of the evolutionary metadynamics method lies in that it is highly suitable for harvesting low-energy metastable structures in addition to the ground state. Those previously proposed candidate structures for the product of cold compression of graphite, bct-C , M, and W-carbon are all easily recovered in a single simulation. More interestingly, we also observed many low-energy structures based on 5+7 or 4+8 topology. The B+D type structure (Fig. 8.7d) observed in the simulation is actually the oC16 structure (sometimes called Z carbon), recently suggested as a candidate for superhard graphite. Since oC16 inherits layers of bct-C
, M, and W-carbon are all easily recovered in a single simulation. More interestingly, we also observed many low-energy structures based on 5+7 or 4+8 topology. The B+D type structure (Fig. 8.7d) observed in the simulation is actually the oC16 structure (sometimes called Z carbon), recently suggested as a candidate for superhard graphite. Since oC16 inherits layers of bct-C and diamond, there is no surprise that its thermodynamic properties are intermediate between these two structures. The 5+7 class of structures shows a larger diversity. In some of these structures, 5-membered rings form pairs, while in others these they are single. The difference of the 5-membered ring pairs’ orientation leads to two allotropes: M-carbon (Fig. 8.7e) and W-carbon (Fig. 8.7f). Some structures can be thought of as combinations of layers of the M-carbon and diamond structures (M+D carbon, as shown in Fig. 8.7g)
and diamond, there is no surprise that its thermodynamic properties are intermediate between these two structures. The 5+7 class of structures shows a larger diversity. In some of these structures, 5-membered rings form pairs, while in others these they are single. The difference of the 5-membered ring pairs’ orientation leads to two allotropes: M-carbon (Fig. 8.7e) and W-carbon (Fig. 8.7f). Some structures can be thought of as combinations of layers of the M-carbon and diamond structures (M+D carbon, as shown in Fig. 8.7g)