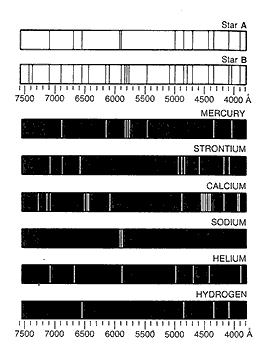

Caption: Emission line spectra in image representation for stellar spectroscopy analysis in black-and-white and NOT color for some reason.
Features:
Note an angstrom (Å) = 10**(-10) m = 0.1 nm. It is a non-standard unit, but often used by spectroscopists and it is also the natural unit for atoms since atoms are of size scale 1 Å.
The range of the horizontal axis is the visible band (fiducial range 0.4--0.7 μm) plus a bit more: 3800--7600 Å.
Yours truly thinks all the atoms are neutral, except the calcium may be ionized (i.e., Ca II) since the Ca II K & H lines (with, respectively, λ = 393.366 nm and λ = 396.847 nm) seem to be present in the image.