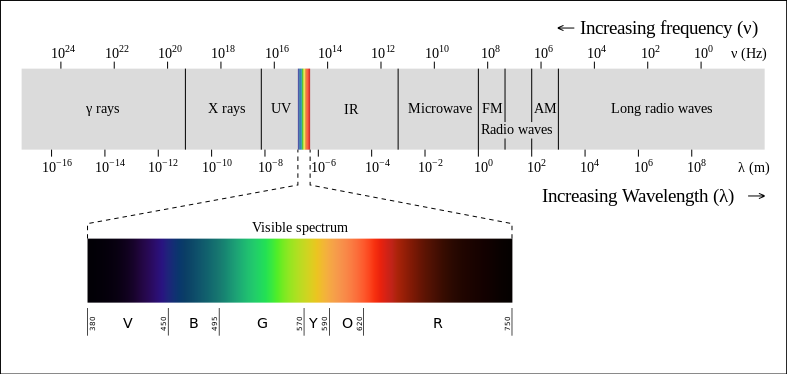
Caption: The electromagnetic spectrum.
Wave Specification: Frequency, Wavelength, Photon Energy, Wavenumber:
Periodic waves can be specified (or characterized) by either wavelength λ or frequency f since they are reciprocals of each other aside from a constant as shown by the generic phase velocity formula fλ=v_phase where v_phase is the phase velocity, the velocity at which a waveform propagates. The 2 specifications have the same information content.
Electromagnetic radiation (EMR) waves are a special case since they can be specified in 4 related ways with the same information content: by wavelength λ, frequency f = c/λ, photon energy E = hf = hc/λ = 1.2398419739(75) eV-μ/λ_μ and wavenumber k = 1/λ, where vacuum light speed c = 2.99792458*10**8 m/s (exact by definition) ≅ 3*10**8 m/s = 3*10**5 km/s ≅ 1 ft/ns, Planck constant h = 6.62607015*10**(-34) J·s = (4.135667696 ...)*10**(-15) eV-s (exact by definition), and the electron-volt (eV) = 1.602176634*10**(-19) J (exact by definition).
Note the most familiar wave specification formula for EMR
fλ=c
applies exactly only in vacuum. Note also photon energy and wavenumber are both just proportional to frequency.Which wave specification you use depends on convenience or convention.
-
To explicate conventional uses:
- wavelength
is conventionally used for the:
- ultraviolet band (fiducial range 0.01--0.4 μm).
- visible band (fiducial range 0.4--0.7 μm).
- infrared band (fiducial range 0.7 μm -- 0.1 cm).
- frequency is conventionally used for the radio band (fiducial range 3 Hz -- 300 GHz = 0.3 THz, 0.1 cm -- 10**5 km) usually using as convenient any of the units:
- photon energy E = hf = hc/λ
= 1.2398419739(75) eV-μ/λ_μ
(where the formulae are the
de Broglie relations
and the electron-volt (eV)
= 1.602176634*10**(-19) J (exact by definition))
is conventionally used for the:
- X-ray band (fiducial range 0.1240--124.0 keV, 0.1--100 Å)
- gamma ray band (fiducial range 1 keV--∞ = 0.001--∞ MeV).
- wavenumber k = 1/λ is also conventionally used for the ultraviolet band (fiducial range 0.01--0.4 μm), visible band (fiducial range 0.4--0.7 μm), and infrared band (fiducial range 0.7 μm -- 0.1 cm) with the common unit being inverse centimeters (cm**(-1). Wavenumber was conventionally used in spectroscopy, but is nowadays becoming obsolete yours truly thinks.
Credit/Permission: ©
Philip Ronan (AKA User:Sakurambo,
2007 /
Creative Commons
CC BY-SA 3.0.
Image link: Wikipedia:
File:EM spectrum.svg.
Local file: local link: electromagnetic_spectrum_wave_specification.html.
File: Electromagnetic Radiation file:
electromagnetic_spectrum_wave_specification.html.