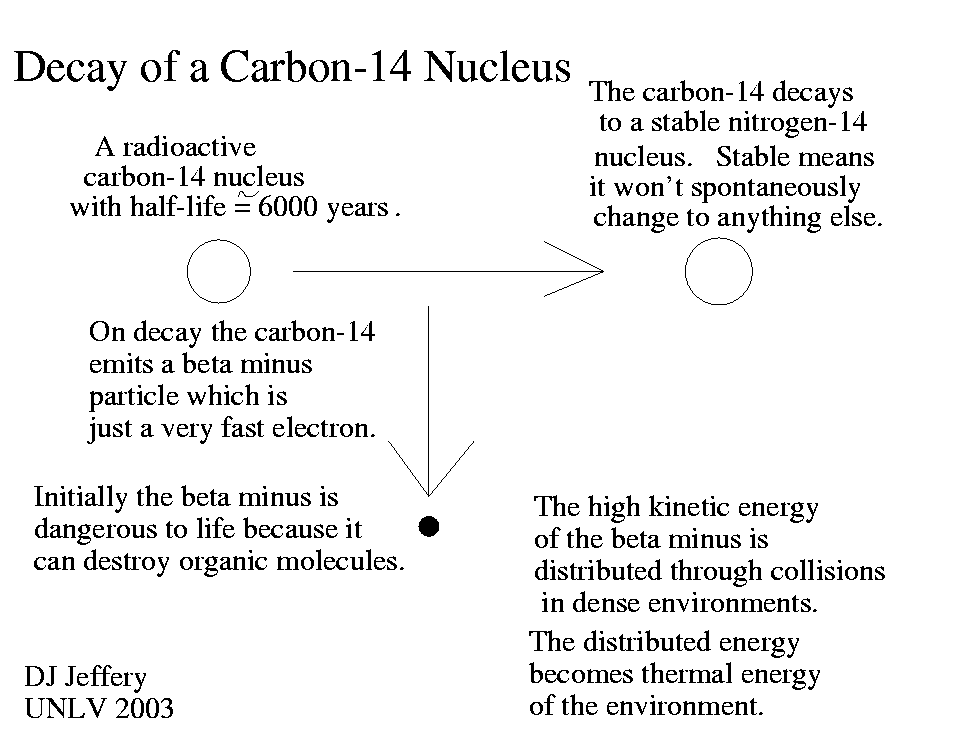
Caption: A cartoon of radioactive isotope (AKA radionuclide) carbon-14 (C-14, radiocarbon, 6 p, 8 n, half-life =5700(30) y, t_e=8220 y) undergoing radioactive decay.
Features:
- C-14 undergoes spontaneously
radioactive decay
(due to weak nuclear force) to
nitrogen-14 (N-14, 7 p, 8, n, stable,
terrestrial abundance 99.636 % out all nitrogen)
by beta decay:
C-14 → N-14 + e**- + ν**(bar) + 0.156476 MeV ,
where the last term is the decay energy in mega-electron-volts (MeV), e**- is negative charge beta particle (just a high kinetic energy electron) and ν**(bar) electron antineutrino. The ejected electron is high kinetic energy which makes it dangerous ionizing radiation. - C-14 is created naturally in the
Earth's atmosphere
by the (n-p) reaction
(a nuclear reaction):
N-14 + n → C-14 + p
where n stands for neutron and p for proton. The neutrons in the Earth's atmosphere are mainly produced by high kinetic energy cosmic rays (which come from space). The ejected proton is just a hydrogen ion (H**(+). - The (approximate) equilibrium C-14 abundance
in the Earth's atmosphere
is ∼ 1/10**12 out all
carbon (C)
atoms.
The equilibrium abundance
is maintained by cosmic ray creation
and spontaneously
radioactive decay destruction.
However, the abundance does vary at bit over
geologic time.
- C-14 is from a
nuclear physics perspective very
different from
carbon-12 (C-12, 6 p, 6 n, stable
terrestrial abundance 98.93 % out all carbon), but from a
chemistry perspective the
2
carbon isotopes are almost identical.
Thus, plants (which get nearly all their carbon from the Earth's atmosphere) have the atmospheric abundance of C-14, and so do all the animals which derive their carbon at some remove from the plants.
- However, when biota
die, their
C-14
radioactively decays away.
The ratio of
C-14
to carbon-12
in dead
organic matter
allows radioactive dating
to infer the time since
death.
- There are uncertainties
in radioactive dating
by C-14 abundance, one of which
is the variation in
the atmospheric abundance of C-14
over geologic time.
Also radioactive dating
can only been done to ∼ 60,000 before present (PB)
since too little C-14 remains from before then.
(see Wikipedia:
Carbon-14: Radiocarbon dating).
- Longer lived radioactive isotopes
(e.g., uranium-238 (U-238, half-life 4.468 Gyr,
t_e=6446 Gyr))
can be used for
the radioactive dating of
rock,
but NOT organic matter.
Image link: Itself.
Local file: local link: radioactive_c14.html.
File: Earth file: radioactive_c14.html.