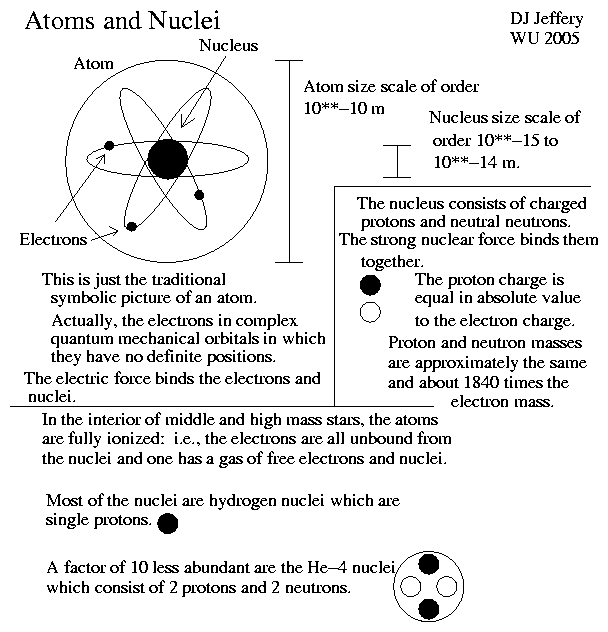
General Caption: Two versions of the standard atom symbol ⚛:
- Image 1 Caption:
A diagram
of a symbolic
lithium-6 (Li-6) isotope atom.
It's based on the
standard atom symbol ⚛ with
electrons in
symbolic
circular
atomic orbitals.
Image 1
also shows symbolically
the 6
nucleons
in the atomic nucleus:
3
protons
and 3
neutrons.
Real atoms
and nuclei do NOT
look like this.
What do real atoms and nuclei look like?
Electrons are thought to be point particles that as dictated by quantum mechanics exist in a superposition of positions, and so effectively form a fuzzy cloud around the nucleus with NO sharp edge. In fact, atoms in actual images look like fuzzy balls because they are, in fact, fuzzy balls. For a real image of atoms, see file atom_gold.html.
Nucleons are nearly point particles: each is thought to be made of 3 quarks. But for most purposes they are effectively point particles that as dictated by quantum mechanics exist in a superposition of positions, and so form a fuzzy cloud (with no sharp edge) at the center of the fuzzy cloud formed by the electrons.
-
Images:
- Credit/Permission: ©
User:AG Caesar,
2018 /
CC BY-SA 4.0.
Image link: Wikimedia Commons:.
- Credit/Permission: ©
David Jeffery,
2005 / Own work.
Image link: Itself.
File: Atomic file: atom_nucleus_symbol.html.