| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Methane, the simplest of saturated hydrocarbons, is an important constituent of gas-giant planets Uranus and Neptune 90. The high-pressure behavior of methane is of extreme importance for fundamental chemistry, as well as for understanding the physics and chemistry of planetary interiors.
The tetrahedral CH molecules are interacting practically only by vdW dispersion forces with each other. In spite of the simplicity of the molecule, the phase diagram of methane is still not well established 91; 92; 93; 94. Different experiments on methane were conducted during the last few decades, resulting in a complex phase diagram drawn by Bini and Pratesi 91. Of the nine solid phases in the diagram, only the structures of phases I, II, and III have been determined, while phases II, III, IV, V, and VI, only exist below 150 K and at moderate pressures. CH
molecules are interacting practically only by vdW dispersion forces with each other. In spite of the simplicity of the molecule, the phase diagram of methane is still not well established 91; 92; 93; 94. Different experiments on methane were conducted during the last few decades, resulting in a complex phase diagram drawn by Bini and Pratesi 91. Of the nine solid phases in the diagram, only the structures of phases I, II, and III have been determined, while phases II, III, IV, V, and VI, only exist below 150 K and at moderate pressures. CH is expected to become chemically unstable and decompose at megabar pressures 95.
is expected to become chemically unstable and decompose at megabar pressures 95.
The high-pressure phases of solid methane A above 5 GPa have been the subject of numerous experimental and theoretical studies, however, the understanding are still incomplete. Bini and Pratesi 91 based on IR and Raman data, proposed a tetragonal crystal structure for the phase A, while high-pressure X-ray powder diffraction experiments suggested that the unit cell contains 21 molecules in the pseudocubic rhombohedral unit cell 93.
We performed structure prediction simulation for CH using experimental cell parameters 94 (a=8.36 at 11 GPa). We indeed found the best structure to possess a rhombohedral symmetry, and this structure was found within 8 generations, and is characterized by the icosahedral packing of methane molecules, and this packing fully explains the unusual number 21 of molecules in the cell: 1 molecule is located in the center of the unit cell, 12 molecules around it form an icosahedron, and the remaining 8 molecules are located above the triangular faces of the icosahedron (Fig. 3.6) and form around the latter. A rhombohedral model, very similar to ours, was recently proposed, on the basis of neutron scattering experiments 92: the only difference is that our model has orientationally disordered molecules (as is also most likely to be the case in reality: furthermore, this model has a lower energy), while Ref. 92 assumed orientationally ordered molecules. The essential icosahedral character of the structure was not mentioned in Ref. 92, but can be clearly seen on close inspection of its results.
using experimental cell parameters 94 (a=8.36 at 11 GPa). We indeed found the best structure to possess a rhombohedral symmetry, and this structure was found within 8 generations, and is characterized by the icosahedral packing of methane molecules, and this packing fully explains the unusual number 21 of molecules in the cell: 1 molecule is located in the center of the unit cell, 12 molecules around it form an icosahedron, and the remaining 8 molecules are located above the triangular faces of the icosahedron (Fig. 3.6) and form around the latter. A rhombohedral model, very similar to ours, was recently proposed, on the basis of neutron scattering experiments 92: the only difference is that our model has orientationally disordered molecules (as is also most likely to be the case in reality: furthermore, this model has a lower energy), while Ref. 92 assumed orientationally ordered molecules. The essential icosahedral character of the structure was not mentioned in Ref. 92, but can be clearly seen on close inspection of its results.
![\includegraphics[scale=1.0]{chapter3/pdf/Fig5.png}](images/img-0202.png)
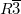 ); Two C sublattices are marked by different colors in 21-molecule cell (non-icosahedral, green; icosahedral, grey)
); Two C sublattices are marked by different colors in 21-molecule cell (non-icosahedral, green; icosahedral, grey)