| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Ice (H O) is an archetypal hydrogen-bonded molecular crystal. The orientations of hydrogen bonds locally obey the well-known ice rules, that is, each oxygen atom is tetrahedrally bonded to four hydrogen atoms, by two strong covalent intra-molecular bonds and two much weaker inter-molecular bonds (hydrogen bonds). Given the enormous number of possibilities of placing and orienting (even under ice rules) water molecules, the prediction of the ice structure is a complex task: according to Maddox(1988), it is still thought to lie beyond the mortal’s ken.
O) is an archetypal hydrogen-bonded molecular crystal. The orientations of hydrogen bonds locally obey the well-known ice rules, that is, each oxygen atom is tetrahedrally bonded to four hydrogen atoms, by two strong covalent intra-molecular bonds and two much weaker inter-molecular bonds (hydrogen bonds). Given the enormous number of possibilities of placing and orienting (even under ice rules) water molecules, the prediction of the ice structure is a complex task: according to Maddox(1988), it is still thought to lie beyond the mortal’s ken.
The normal crystalline form of ice, ice I , is disordered and has hexagonal symmetry, with oxygen atoms arranged in a hexagonal diamond motif (a cubic diamond-type ice I
, is disordered and has hexagonal symmetry, with oxygen atoms arranged in a hexagonal diamond motif (a cubic diamond-type ice I is also known experimentally) with randomly oriented hydrogen bonds. In experiment 84; 85, ice XI (ordered version of ice I
is also known experimentally) with randomly oriented hydrogen bonds. In experiment 84; 85, ice XI (ordered version of ice I ), found to be the most stable polymorph at 1 atm and low temperatures, but the transformation from disordered ice I
), found to be the most stable polymorph at 1 atm and low temperatures, but the transformation from disordered ice I to ordered ice XI is kinetically hindered, and this is why special approaches are needed for experimental preparation of ice XI 84.
to ordered ice XI is kinetically hindered, and this is why special approaches are needed for experimental preparation of ice XI 84.
With variable-cell USPEX simulations for a 4-molecules cell at 1 atm, we indeed identify ice XI as the most stable polymorph (Fig. 3.4a). This structure was found within just 4 generations, after relaxing  160 structures. Fig. 3.5 shows how the lowest energy changed from generation to generation in our calculation. This purely quantum-mechanical calculation required less than 1 day on 8 cores of a Dell XPS desktop PC. Apart from ice XI, we found several remarkable structures in the same run.
160 structures. Fig. 3.5 shows how the lowest energy changed from generation to generation in our calculation. This purely quantum-mechanical calculation required less than 1 day on 8 cores of a Dell XPS desktop PC. Apart from ice XI, we found several remarkable structures in the same run.
The ordered version of ice I 86, a tetragonal phase with a cubic diamond-type oxygen sublattice (Fig. 3.4b, it has two type of Wyckoff positions, O(0,0.5,0.0006), H(0.1839,0.5,0.1000)), The cubic-like structure was found to be energetically competitive with ice XI. At both the GGA and GGA+D levels of theory, its energy is only 2 meV/molecule above that of ice XI. We have also found an interesting low-energy metastable polymorph (Fig. 3.4c), where the oxygen sublattice has topology of the hypothetical bct4 allotrope of carbon 87; 88. The bct4-like structure of ice was also found from molecular dynamics simulation of the water’s adsorption on the surface of hydroxylated
86, a tetragonal phase with a cubic diamond-type oxygen sublattice (Fig. 3.4b, it has two type of Wyckoff positions, O(0,0.5,0.0006), H(0.1839,0.5,0.1000)), The cubic-like structure was found to be energetically competitive with ice XI. At both the GGA and GGA+D levels of theory, its energy is only 2 meV/molecule above that of ice XI. We have also found an interesting low-energy metastable polymorph (Fig. 3.4c), where the oxygen sublattice has topology of the hypothetical bct4 allotrope of carbon 87; 88. The bct4-like structure of ice was also found from molecular dynamics simulation of the water’s adsorption on the surface of hydroxylated  -cristobalite 89. Proton ordering lowers its symmetry from I4/mmm to Cm. Atoms in this structure has eight types of Wyckoff positions (O1(0,0,0), O2(0.3691,0,0.7197), O3(0.6647,0.3192,0.3605), H1(0.7683,0,0.8871), H2(0.1317,0,0,8908), H3(0.4705, 0.2691, 0.3567), H4(0.0923,0.8787,0.2133), H5(0.3018,0.0751,0.6030)).
-cristobalite 89. Proton ordering lowers its symmetry from I4/mmm to Cm. Atoms in this structure has eight types of Wyckoff positions (O1(0,0,0), O2(0.3691,0,0.7197), O3(0.6647,0.3192,0.3605), H1(0.7683,0,0.8871), H2(0.1317,0,0,8908), H3(0.4705, 0.2691, 0.3567), H4(0.0923,0.8787,0.2133), H5(0.3018,0.0751,0.6030)).
![\includegraphics[scale=0.6]{chapter3/pdf/Fig3.png}](images/img-0199.png)
 , space group Cmc2
, space group Cmc2 , a=4.338 , b=7.554 , c=7.094 ; b) tetragonal phase, derived from ice I
, a=4.338 , b=7.554 , c=7.094 ; b) tetragonal phase, derived from ice I , space group I4
, space group I4 md, a=4.415 , c=6.008 ; c) bct-4 like ice, space group Cm, a=4.472 , b=10.451 , c=5.744 ,
md, a=4.415 , c=6.008 ; c) bct-4 like ice, space group Cm, a=4.472 , b=10.451 , c=5.744 , 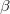 =111.3
=111.3 .
.![\includegraphics[scale=0.6]{chapter3/pdf/Fig4.png}](images/img-0201.png)