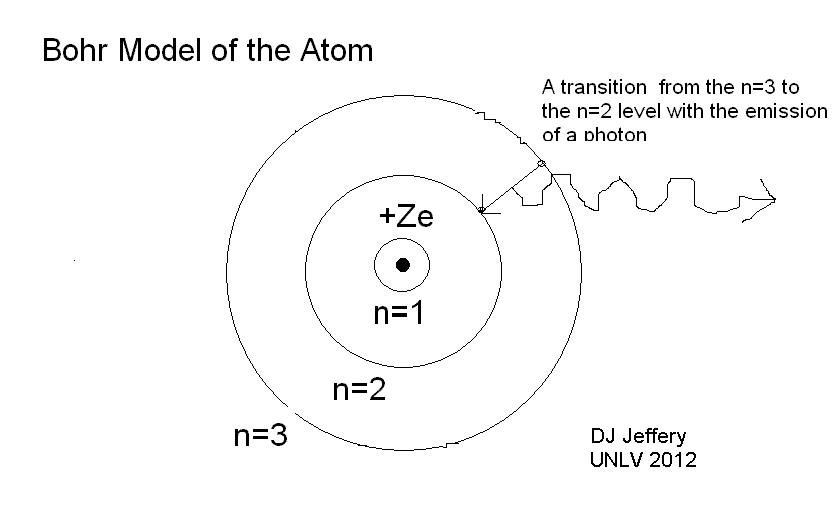
Caption: An ABSTRACT diagram of a hydrogen atom changing energy levels and emitting a photon. In general, a change in energy levels is called a atomic transition.
Features:
- Atoms don't really look like this.
Atoms can be imaged directly in various ways. But they do NOT have sharp edges, and so they look like fuzzy balls. In general, a real image of an atom does NOT give a clear picture of its structure in any direct way.
- The ABSTRACT diagram
derives from the
Bohr atom which
was a model of the
hydrogenic atom (i.e.,
a hydrogen-like atom)
introduced in 1913 by
Niels Bohr (1885--1962).
The Bohr atom is historically important
in the development of
quantum mechanics, but it is
a wrong model.
But it is right in that there are NOT a continuum of energy states allowed for the electrons that surround the atomic nucleus which for hydrogen (the simplest of all atomic nuclei) is a single proton represented by the central dot in the diagram. A hydrogen atom also has only one electron represented by a small dot in the diagram.
Instead of a continuum of energy states, there is only a discrete set with discrete energies. This is main reason why we call quantum mechanics quantum mechanics: the energy states are QUANTIZED.
- For atoms, we call the
energy states
energy levels.
The quantized energy levels in the ABSTRACT diagram are represented by a quantized set of circular orbits.
The larger the circular orbit, the higher the energy of the energy level.
The smallest circle represents the ground state, the lowest energy energy level allowed by quantum mechanics.
The Bohr atom did posit actual circular orbits, but that turned out to be WRONG.
- To emphasize, the
circular orbits
are NOT the real appearance of the
energy levels.
In fact, each energy level is a spread out density distribution for an electron. The electron exists in a continuum superposition of positions with the amount of it at any point being determined by the density distribution.
The electron is usually only one energy level at at time---but it can be in a superposition of energy levels.
-
When the electron changes
energy levels
(i.e., makes an
atomic transition),
energy
is emitted or absorbed.
- A common way for this to happen is by
the emission or absorption of a photon:
the quantum particle of
electromagnetic radiation.
The energy of the emitted or absorbed photon is determined by the conservation of energy.
An absorption process requires an incident photon.
- An emission can be a
spontaneous emission
or can be stimulated emission caused by
an incident
photon.
- There is NO
spontaneous emission
NOR stimulated emission
from the lowest energy level
the ground state.
In the ground state, the atom simply has no REMOVABLE energy.
It does have an IRREMOVABLE zero-point energy dictated by quantum mechanics.
- Since the energy levels are quantized,
only certain energies are allowed for the
emitted or absorbed photons.
This means only certain frequencies and wavelengths are allowed for the emitted or absorbed photons.
- If ΔE is the change in energy of the
atom for
an emission and also thereby the
energy of the
emitted photon,
the frequency ν of the emitted
photon
is determined by the
de Broglie relation
ΔE=hν, where the h is the
Planck constant h = 6.626070040(81)*10**(-34) J-s
which is a fundamental constant
of nature.
Wavelength is given by de Broglie relation ΔE=hc/λ, where λ is wavelength.
- Every
atom and
molecule
has a unique set of quantized
energy levels, and so every
atom and
molecule
has a unique discrete spectrum of
frequencies/wavelengths of
emission/absorption.
These spectra
are called line spectra.
The study/analysis of line spectra
is called spectroscopy.
Image link: Itself.
Local file: local link: atom_diagram_abstract.html.
File: Atomic file: atom_diagram_abstract.html.