| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Fig 9.4 shows the predicted Gibbs free energy of formation of Xe-O compounds at different temperatures and 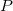 = 100 GPa. It can be clearly seen that XeO and XeO
= 100 GPa. It can be clearly seen that XeO and XeO are thermodynamically stable at 100 GPa. The thermal stability of XeO
are thermodynamically stable at 100 GPa. The thermal stability of XeO increases with temperature.
increases with temperature.
![\includegraphics[scale=0.5]{pdf/100GPa.png}](images/img-0341.png)
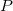 = 100 GPa. For clarity, the Gibbs free energies of formation were shifted by -0.1, -0.2, and -0.3 eV/atom at 1000, 2000, and 3000 K, respectively.
= 100 GPa. For clarity, the Gibbs free energies of formation were shifted by -0.1, -0.2, and -0.3 eV/atom at 1000, 2000, and 3000 K, respectively.