| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Light weight metal borohydrides have recently received much attention owing to their high gravimetric and volumetric hydrogen densities compared to other complex hydrides 123; 124; 125. Of these, magnesium borohydride, Mg(BH )
) , is a prominent lightweight solid-state hydrogen storage material with a theoretical hydrogen capacity of 14.8 wt %. Mg(BH
, is a prominent lightweight solid-state hydrogen storage material with a theoretical hydrogen capacity of 14.8 wt %. Mg(BH )
) at ambient condition has been extensively studied. To improve the reversible hydrogen absorption or desorption kinetics or get new metastable polymorphs, recent interest are foucusing on the stabilization of the high-pressure phase of Mg(BH
at ambient condition has been extensively studied. To improve the reversible hydrogen absorption or desorption kinetics or get new metastable polymorphs, recent interest are foucusing on the stabilization of the high-pressure phase of Mg(BH )
) at ambient pressure. For example,
at ambient pressure. For example, 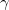 -Mg(BH
-Mg(BH )
) is one of the hydrogen-richest solids, reported to be capable of storing guest species, such as hydrogen 126. Most recently, new
is one of the hydrogen-richest solids, reported to be capable of storing guest species, such as hydrogen 126. Most recently, new 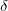 ,
,  , and
, and  phases of Mg(BH
phases of Mg(BH )
) were successfully synthesized under pressure 126. Many of them turned out to retain their structure upon decompression to ambient conditions. Crystal structures of
were successfully synthesized under pressure 126. Many of them turned out to retain their structure upon decompression to ambient conditions. Crystal structures of 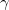 and
and 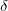 phases were, apparently convincingly, resolved using powder x-ray diffraction data obtained with synchrotron radiation 126. Unexpectedly, theoretical phonon calculations showed the
phases were, apparently convincingly, resolved using powder x-ray diffraction data obtained with synchrotron radiation 126. Unexpectedly, theoretical phonon calculations showed the 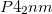 structure (
structure (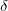 phase) to be dynamically unstable at ambient pressure, which means that the exact crystal structure of
phase) to be dynamically unstable at ambient pressure, which means that the exact crystal structure of 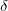 phase is still unresolved, even for such a simple structure only with 22 atoms per cell, and still less for the unknown
phase is still unresolved, even for such a simple structure only with 22 atoms per cell, and still less for the unknown  and
and  phases 126. Therefore, the polymorphism and phase diagram of this important compound require further investigation.
phases 126. Therefore, the polymorphism and phase diagram of this important compound require further investigation.