| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
Apart from molecular crystals, this new approach is also applicable to inorganic crystals with complex ions or clusters. Below is an illustrations on Elemental boron.
Boron, located in a unique position of Periodic Table, is an element of chemical complexity due to the subtle balance between metallic and insulating states. All known structures of boron contain icosahedral B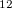 clusters. Recent experiment 12 found a new phase of pure boron (
clusters. Recent experiment 12 found a new phase of pure boron (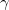 -B
-B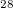 ) at pressures above 10-12 GPa, and its structure was solved using USPEX with fixed experimental cell dimensions 12. Surprisingly,
) at pressures above 10-12 GPa, and its structure was solved using USPEX with fixed experimental cell dimensions 12. Surprisingly, 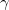 -B
-B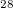 showed different chemistry compared with all the other elemental boron polymorphs. In the
showed different chemistry compared with all the other elemental boron polymorphs. In the 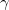 -B
-B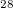 structure (Fig. 3.12b), the centers of the B
structure (Fig. 3.12b), the centers of the B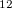 icosahedra form a distorted cubic close packing as in
icosahedra form a distorted cubic close packing as in  -
-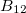 (Fig. 3.12a); but with all octahedral voids are occupied by
(Fig. 3.12a); but with all octahedral voids are occupied by 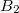 pairs. The
pairs. The 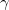 -B
-B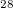 structure resembles a NaCl-type structure, with the B
structure resembles a NaCl-type structure, with the B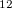 icosahedra and B
icosahedra and B pairs as ‘anions’ and ‘cations’. Finding this structure without fixing cell parameters was reported to be exceedingly difficult 120, but latest methodological developments enable this (Lyakhov et al., unpublished). However, the problem can be made very simple if we recall that all boron phases contain B
pairs as ‘anions’ and ‘cations’. Finding this structure without fixing cell parameters was reported to be exceedingly difficult 120, but latest methodological developments enable this (Lyakhov et al., unpublished). However, the problem can be made very simple if we recall that all boron phases contain B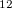 icosahedra. Here we treated B
icosahedra. Here we treated B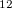 icosahedral and B
icosahedral and B pairs as separate rigid units, and performed structure prediction runs at different numbers of B
pairs as separate rigid units, and performed structure prediction runs at different numbers of B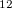 and B
and B units (2:1, 1:1, 2:2, 2:4, etc) at ambient conditions. We could easily find
units (2:1, 1:1, 2:2, 2:4, etc) at ambient conditions. We could easily find 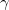 -B
-B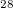 within 2 - 3 generations or
within 2 - 3 generations or  100 structural relaxations. Meanwhile, we observed a set of low-energy and chemically interesting structures with different proportions of B
100 structural relaxations. Meanwhile, we observed a set of low-energy and chemically interesting structures with different proportions of B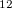 and B
and B . For instance, the novel metallic phase B
. For instance, the novel metallic phase B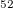 with the Pnn2 symmetry (Fig. 3.12c) was calculated to be only 12 meV/atom higher than
with the Pnn2 symmetry (Fig. 3.12c) was calculated to be only 12 meV/atom higher than 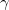 -B
-B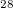 at atmosphere pressure. The value is lower than those of the experimentally observed phases (such as the T-50 phase 121) and this example shows that our method can be used for even non-molecular and inorganic solids that contain clusters or complex ions.
at atmosphere pressure. The value is lower than those of the experimentally observed phases (such as the T-50 phase 121) and this example shows that our method can be used for even non-molecular and inorganic solids that contain clusters or complex ions.
![\includegraphics[scale=0.5]{chapter3/pdf/Fig12}](images/img-0222.png)
 -B
-B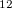 ; b)
; b) 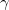 -B
-B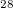 ; c) novel metastable B
; c) novel metastable B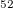 phase, space group Pnn2, a=8.868 , b=8.777 , c=5.000 .
phase, space group Pnn2, a=8.868 , b=8.777 , c=5.000 .