Image Questions
Image Answers
Caption: "Democritus (ca. 460--ca. 370 BCE) meditating on the seat of the soul (1868) by Leon-Alexandre Delhomme (1841--1893 or 1895)."
Democritus was foremost exponent of atomism among the ancient Greeks
Credit: User:Jean-Louis Lascoux.
Image linked to Wikipedia.
Permission: Use under GNU Free Documentation License.
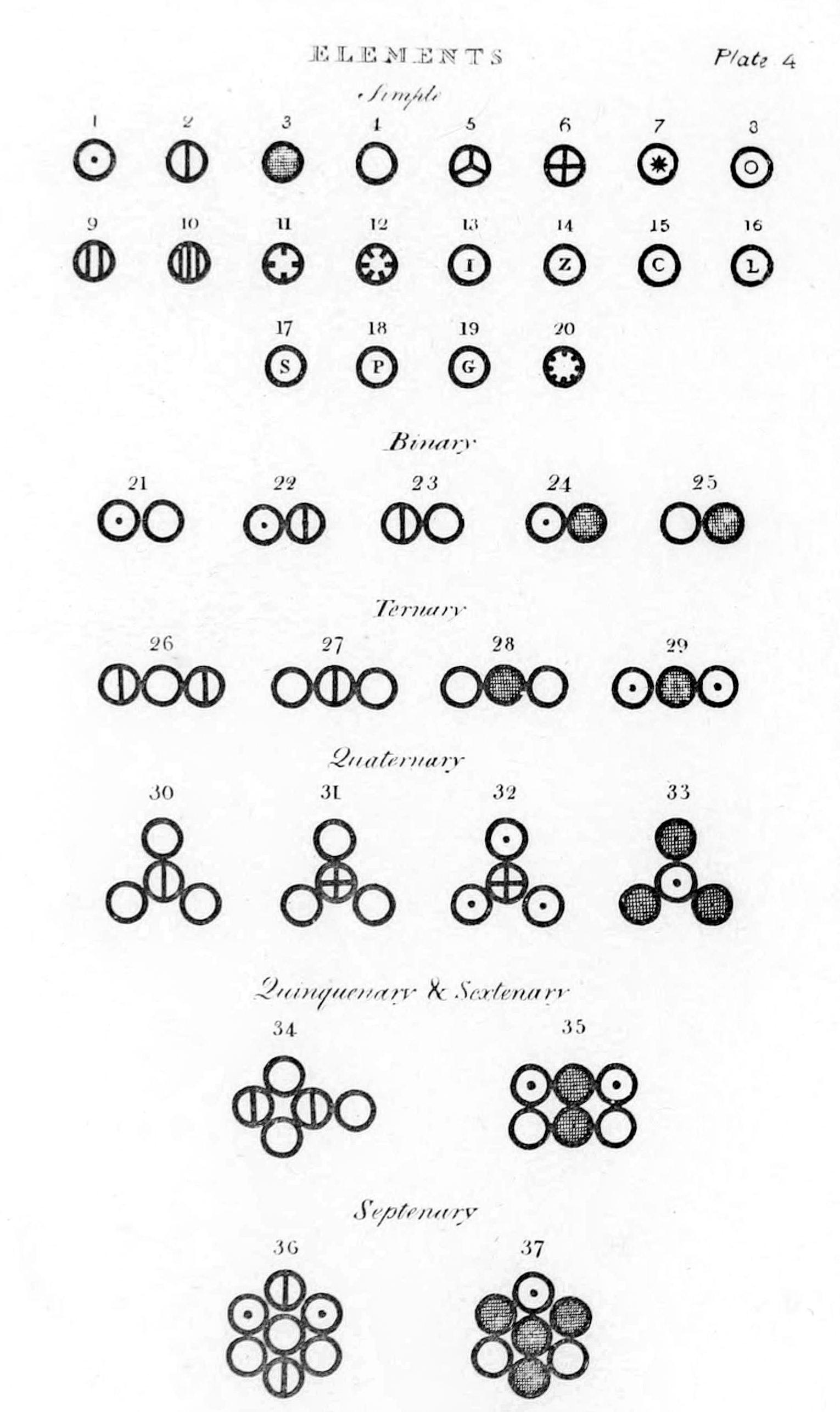
Caption: `A scan of the first page of John Dalton's (1766--1844) "A New System of Chemical Philosophy", published in 1808. Please do not "update" the list with modern spellings. This is a historic list and the old spellings are intentional. Yes, it's "carbone", not "carbon".'
The relative atomic masses of the atoms, but not molecules shown in the figure.
1. Hydrogen, its relative weight 1 2. Azote 5 3. Carbone or charcoal 5 4. Oxygen 7 5. Phosphorous 9 6. Sulphur 13 7. Magnesia 20 8. Lime 23 9. Soda 28 10. Potash 42 11. Strontites 46 12. Barytes 68 13. Iron 38 14. Zinc 56 15. Copper 56 16. Lead 95 17. Silver 100 18. Platina 100 19. Gold 140 20. Mercury 167Credit: John Dalton (1766--1844), User:haade.
Permission: Public domain at least in USA.
Image linked to Wikipedia.

Caption: "Reproduced from the book of Jean Baptiste Perrin (1870--1942), `Les Atomes', three tracings of the motion of colloidal particles of radius 0.53 Ám, as seen under the microscope, are displayed. Successive positions every 30 seconds are joined by straight line segments (the mesh size is 3.2 Ám)."
Because, the observations are 30 seconds apart, the motion between the observations is NOT seen in detail and is only crudely approximated by the straight line segments.
The colloidal particles (which are macroscopic) are driven in a random walk by random collisions with molecules.
The collodial particle motion is called Brownian motion after the effective discoverer of the phenomenon Robert Brown (1773--1858) in 1827.
Credit: Jean Baptiste Perrin (1870--1942), User:MiraiWarren.
Permission: Public domain at least in USA.
Image linked to Wikipedia.

Caption: "Beam of electrons moving in a circle in a magnetic field (cyclotron motion). Lighting is caused by exitation of atoms of gas in a bulb."
Electrons were definitively discovered as new particles in 1896 by J. J. Thomson (1856--1940 and colleagues.
They could be produced in beams from hot metal surfaces and directed by electric fields and magnetic fields.
Electrons had negative charge and were ubiquitous in all materials.
This suggested electrons were constituents of atoms and that atoms needed to have positive constituents as well in order to be mainly neutral overall.
The fact that atoms had constituents meant they could not be the simple, uncutable entities the ancient atomists hat thought.
Credit: User:Sfu AKA Marcin Bialek.
Permission: Use under GNU Free Documentation License.
Image linked to Wikipedia.

Caption: A of an atom changing it's energy state and emitting a photon.
Atoms don't really look like this.
This is Bohr atom, a model invented by Niels Bohr (1885--1962) in 1913.
The Bohr atom correctly incorporated the recently discovered atomic nucleus.
But essentially, it is a wrong model. It is still used for illustrative purposes.
The center of the ``orbits'' is the atomic nucleus.
There is only one electron shown and it can be in a discrete set of ``orbits'' ONLY.
These ``orbits'' are the quantized energy states or levels of the atom.
When the electron changes energy state a photon is emitted or absorbed.
The emitted or absorbed photon maintains the conservation of energy.
An absorption process requires an incident photon.
An emission process can happen spontaneously or can be stimulated to happen by an incident photon.
But there is NO spontaneous emission from the lowest energy level---this the ground level in quantum mechanics jargon.
Since the energy levels are quantized, only certain energies of photons are allowed.
This means only certain frequencies and wavelengths are allowed.
-
Delta E is the change in energy. v is the frequency of the emission.
h is the Planck constant which is
a fundamental constant of nature.
The energy levels are pictured as circular orbits, but nowadays we know that this is not really true.
The energy levels must be represented by distributions of positions since the electrons are in a continuum superposition positions.
But early model of the atom, the Bohr atom, did posit circular orbits.
Credit: User:JabberWok.
Image linked to Wikipedia.
Permission: Use under GNU Free Documentation License.

Caption: Cartoon of gas molecules motion at finite temperature.
Credit: User:Greg L.
Image linked to Wikipedia.
Permission: Use under GNU Free Documentation License

A Helium-4 (He-4) atom and nucleus.
The size scale of the atom is about an angstrom (10**(-10) m).
The grey shading represents the electron distribution.
There are only two electrons, but they exist in superposition of locations.
Quantum mechanical particles are fractionally in a continuum of places at once. This is one of the things that makes quantum mechanics so tricky.
The scaled up region represents the atomic nucleus which consists of two protons and two neutrons bound together by the strong nuclear force.
The positively charged protons repel each other by the electromagnetic force, but the strong nuclear force overcomes this.
The proton and two neutron actually exist in a superposition of locations too---the illustration is only meant to be symbolical.
The size scale of the nucleus is about a fermi (10**(-15) m).
So the nucleus is about 10**5 times smaller than the atom, but it is about 4000 times more massive.
The negatively charged electrons are bound to the nucleus by attraction to the positively charged protons.
Credit: Yzmo.
Image linked to Wikipedia.
Permission: Use under GNU Free Documentation License.
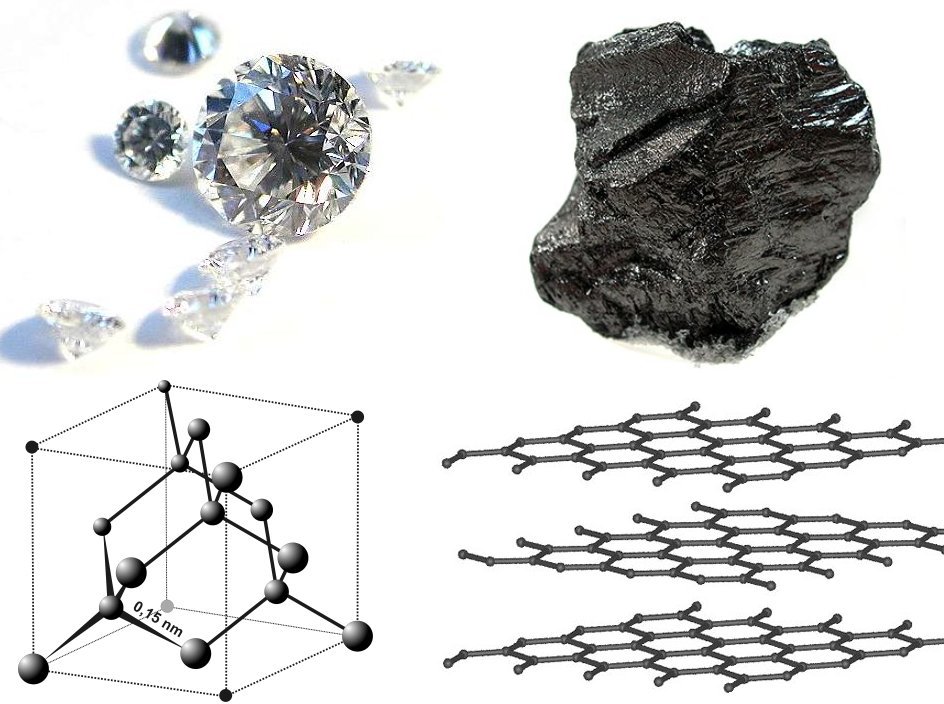
Caption: "Diamond and graphite samples with their respective structures."
Credit: User:Itub.
Permission: Use under GNU Free Documentation License.
Image linked to Wikipedia.

Caption: "Buckyball with isosurface of ground state electron density (calculated with DFT, the CPMD code). File was rendered using VMD."
Fullerenes are molecules composed entirely of carbon. Apparently, carbon nanotubes are considered fullerenes, but I don't see how one can call something without a definite number of atoms a molecule.
Molecular carbon states are distinct from graphite and fullerene is the carbon-60 molecule also called buckminsterfullerene or buckyballs.
The equilibrium position of the bonded carbon atoms are on pentagons and hexagons like the vertex points of standard soccer ball.
In the illustration, the ``wire mesh'' represents a isodensity surface for electron density in the ground state. The electron density decreases away from the C-60 and in the inner region.
The electrons provide the bonding of the carbon atoms.
Fullerenes have interesting properties. You can trap other particles on the inside for example.
Credit: User:Itamblyn.
Image linked to Wikipedia.
Permission: Use under GNU Free Documentation License.
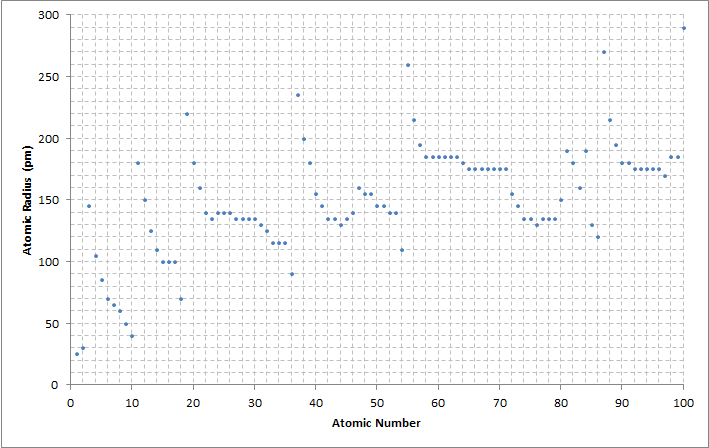
Caption: "A graph comparing the calculated atomic radius of elements with atomic numbers 1--100. Accuracy of ▒5 pm. Data is from the Wikipedia: Atomic Radius page and US Government sites."
Atomic radius varies by a factor of roughly 10 from of order 0.3 angstroms to of order 3 angstroms.
If one just looks at the tightly bound noble gases, atomic radius varies by a factor of about 6 from about 0.3 angstroms (for helium, Z=2) to about 1.5 angstroms (for radon, Z=86).
Neither of these variations suggests any simple trend to me.
One can try a simpleminded model for the variation of atomic radius with atomic number Z.
Assume each electron needs about the same amount of volume for itself. Then atomic volume V goes as atomic number Z, and thus atomic radius goes as Z**(1/3). (Actually, the argument for the model is not valid in quantum mechanics, but a valid quantum mechanics argument can be given.)
Fitting the model with r=1.5 angstroms for Z=50, I get
____________________________ Z r (angstroms) ____________________________ 1 0.407 3 0.587 10 0.877 30 1.27 50 1.50 75 1.72 86 1.80 100 1.89 ____________________________The overall fit is not bad, but it is not great either---and it can't be since the radius model doesn't account for the shell structure of the electron configuration, and so can't get the non-monotonic behavior.
Credit: User:Dot145
Image linked to Wikipedia.
Permission: Licensed under the Creative Commons Attribution ShareAlike 2.5.

Caption: "Crystal structure of sodium chloride (NaCL) (AKA table salt) with coordination polyhedra".
The sodium atoms are grey and the chlorine atoms are green.
Credit: User:Solid State.
Permission: Public domain at least in USA.
Image linked to Wikipedia.
Videos
-
Hydrogen economy videos:
- The Promise and Challenge of the Hydrogen Economy Good. Too long for the classroom.
- Artificial Leaf at MIT The artifical leaf is an example of artificial photosynthesis. In the case of the artificial leaf, one has specifically photocatalytic water splitting or photoelectrolysis. It's not quite as bad as watching paint dry. Suitable for the classroom.
- Hindenburg Disaster Footage (With Herbert Morrison Commentary) The full show. Too long for classroom.
- The Hindenburg Explosion Newsreel style. Suitable for classroom.
-
Nuclear bomb videos
- Tsar Bomba - Largest Nuclear Device Ever Tested (50MT) It's a lot like Dr. Strangelove (1964) for real.
- Atomic Bomb Footage Light heat flash before the shock wave arrives. Short enough for classroom.
- Christmas Island Atomic Bomb Test Short enough for classroom.
