| Crystal Structure Prediction and its Application in Earth and Materials Sciences |
We have performed structure prediction simulations for the Xe-O system for the compositions of XeO, XeO , XeO
, XeO , XeO
, XeO at 5, 50, 100, 120, 150, 180, 200 and 220 GPa. Our calculation at 5 GPa yielded lowest-enthalpy structures that always contained the O
at 5, 50, 100, 120, 150, 180, 200 and 220 GPa. Our calculation at 5 GPa yielded lowest-enthalpy structures that always contained the O molecules, indicating the tendency for segregation of the elements, and indeed at 5 GPa decomposition was found to be energetically favorable. This suggests that the reaction observed by Sanloup et al. 149; 150 at 0.7 to 10 GPa was an entropically driven incorporation of Xe impurities into the structure of SiO
molecules, indicating the tendency for segregation of the elements, and indeed at 5 GPa decomposition was found to be energetically favorable. This suggests that the reaction observed by Sanloup et al. 149; 150 at 0.7 to 10 GPa was an entropically driven incorporation of Xe impurities into the structure of SiO , rather than enthalpically-driven formation of a stoichiometric xenon silicate or oxide. Indeed, solid solutions and point defects are stabilized by entropy 160.
, rather than enthalpically-driven formation of a stoichiometric xenon silicate or oxide. Indeed, solid solutions and point defects are stabilized by entropy 160.
In order to evaluate the thermodynamical stability for compounds with different chemical compositions, we established the so called convex hull for Xe-O system. When building the convex hull, we used the structures of the 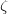 -phase 161 and
-phase 161 and  -phase 162 for the reference of element oxygen, and the fcc 163 and hcp 164 structures for the reference of elemental xenon GPa. Our detailed calculations at and above 100 GPa indeed found a number of oxides stable against decomposition into the elements. The most promising structures and compositions were investigated further. Gibbs free energy of formation of these oxides at high P, T conditions is shown in Fig. 5.1. At 0 K, XeO, XeO
-phase 162 for the reference of element oxygen, and the fcc 163 and hcp 164 structures for the reference of elemental xenon GPa. Our detailed calculations at and above 100 GPa indeed found a number of oxides stable against decomposition into the elements. The most promising structures and compositions were investigated further. Gibbs free energy of formation of these oxides at high P, T conditions is shown in Fig. 5.1. At 0 K, XeO, XeO and XeO
and XeO at high P become thermodynamically stable. We also performed quasi-harmonic free energy calculations with phonon spectra derived by the finite-displacement method 67 to assess the effect of temperature on their stability (Fig. 5.1b). Clearly, only minor quantitative changes occur when temperature is explicitly considered, and XeO, XeO
at high P become thermodynamically stable. We also performed quasi-harmonic free energy calculations with phonon spectra derived by the finite-displacement method 67 to assess the effect of temperature on their stability (Fig. 5.1b). Clearly, only minor quantitative changes occur when temperature is explicitly considered, and XeO, XeO and XeO
and XeO remain thermodynamically stable also at high T.
remain thermodynamically stable also at high T.
![\includegraphics[scale=0.6]{chapter5/pdf/fig1.png}](images/img-0254.png)
 , XeO
, XeO , O
, O .
.Reactions |
|
|
||
Xe + 1/2 O |
|
XeO |
-0.388 |
-1.46 |
Xe + O |
|
XeO |
-0.626 |
-3.11 |
Xe + 3/2 O |
|
XeO |
-0.689 |
-5.52 |
XeO + 1/2 O |
|
XeO |
-0.239 |
-1.65 |
XeO + O |
|
XeO |
-0.301 |
-4.06 |
XeO |
|
XeO |
-0.062 |
-2.41 |
Fig. 5.2d shows the enthalpy of formation of all the Xe oxides as a function of pressure. Below 83 GPa all xenon oxides are unstable. At 83 GPa, XeO-Pbcm becomes stable, followed by XeO -P2
-P2 /c above 102 GPa and XeO
/c above 102 GPa and XeO -P4
-P4 /mnm above 114 GPa. There is a clear trend of increasing the oxidation number of Xe on increasing pressure.
/mnm above 114 GPa. There is a clear trend of increasing the oxidation number of Xe on increasing pressure.
![\includegraphics[scale=0.7]{chapter5/pdf/fig2.png}](images/img-0258.png)
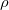 = 0.85) on the Xe-O chain; b) Crystal structure of XeO
= 0.85) on the Xe-O chain; b) Crystal structure of XeO (P2
(P2 /c) structure at 120 GPa, and its ELF picture (
/c) structure at 120 GPa, and its ELF picture (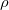 = 0.85) on the XeO
= 0.85) on the XeO square. c) crystal structure of XeO
square. c) crystal structure of XeO (Pmmn) Structure at 200 GPa, and its ELF picture (
(Pmmn) Structure at 200 GPa, and its ELF picture (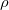 = 0.82) on XeO
= 0.82) on XeO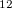 anticuboctahedra; d) Enthalpies of formation (solid lines) and enthalpies of decomposition into other xenon oxides (dashed lines) of all stable xenon oxides. Stability fields are delineated by colors (at 198 GPa, P2
anticuboctahedra; d) Enthalpies of formation (solid lines) and enthalpies of decomposition into other xenon oxides (dashed lines) of all stable xenon oxides. Stability fields are delineated by colors (at 198 GPa, P2 /c XeO
/c XeO transforms to a Cmcm structure, and C2/c-XeO
transforms to a Cmcm structure, and C2/c-XeO transforms to Pmmn phase, i.e., just at the edge of the graph).
transforms to Pmmn phase, i.e., just at the edge of the graph).A simple and clear analysis of chemical bonding can be done using the electron localization function (ELF) 165. The ELF gives information about the valence electron configuration of an atom in a compound. States with closed shell electron configurations(Xe , 5
, 5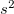 5
5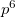 , and Xe
, and Xe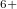 , 5
, 5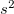 ) will exhibit a spherical ELF distribution, whereas open shell states (Xe
) will exhibit a spherical ELF distribution, whereas open shell states (Xe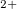 , Xe
, Xe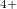 ) will not. For Xe
) will not. For Xe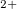 one p-orbital is empty and the ELF will have a toroidal shape; likewise, Xe
one p-orbital is empty and the ELF will have a toroidal shape; likewise, Xe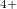 can be formed by the removal of two p-orbitals and the ELF will show a two-lobe maximum corresponding to the shape of the lone p-electron pair.
can be formed by the removal of two p-orbitals and the ELF will show a two-lobe maximum corresponding to the shape of the lone p-electron pair.
The most stable structure of XeO at 100 GPa has space group Pbcm and 8 atoms in the unit cell. As shown in Fig. 5.2a, Xe atoms are in a twofold (linear) coordination and Xe-O bonds form chains, with O-Xe-O angles of 175.6 and Xe-O-Xe angles of 112.6
and Xe-O-Xe angles of 112.6 . The alternating Xe-O bond lengths are 2.0 and 2.1 . The ELF picture (Fig. 5.2a) shows a toroidal maximum of ELF around each Xe atom, exactly what one should expect for Xe
. The alternating Xe-O bond lengths are 2.0 and 2.1 . The ELF picture (Fig. 5.2a) shows a toroidal maximum of ELF around each Xe atom, exactly what one should expect for Xe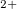 state. Above 145 GPa XeO undergoes a phase transformation and forms a structure with the space group P2
state. Above 145 GPa XeO undergoes a phase transformation and forms a structure with the space group P2 /m. Xe1 connects to four oxygens and has square coordination, and forms thus the same chains as in XeO
/m. Xe1 connects to four oxygens and has square coordination, and forms thus the same chains as in XeO (suggesting that Xe1 atoms are in the tetravalent Xe
(suggesting that Xe1 atoms are in the tetravalent Xe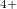 state), whereas Xe2 can be described as neutral and not bonded to other atoms by any significant bonds. The presence of neutral non-bonded atoms in this structure is energetically favourable as it increases its packing density.
state), whereas Xe2 can be described as neutral and not bonded to other atoms by any significant bonds. The presence of neutral non-bonded atoms in this structure is energetically favourable as it increases its packing density.
For XeO , the stable structure above 102 GPa has space group P2
, the stable structure above 102 GPa has space group P2 /c and 24 atoms in the unit cell. Xenon atoms have a slightly non-planar square coordination and the structure consists of 1D-ribbons of edge-sharing XeO
/c and 24 atoms in the unit cell. Xenon atoms have a slightly non-planar square coordination and the structure consists of 1D-ribbons of edge-sharing XeO -squares (Xe-O distances are 2.0 and 2.1 ), with four Xe-O bonds and two lone-pair maxima forming an octahedron, consistent with the geometry proposed by recent experiment 146. Just as in XeO, there are no peaks visible in the ELF isosurface along the Xe-O bonds (Fig. 5.2b). Above 198 GPa XeO
-squares (Xe-O distances are 2.0 and 2.1 ), with four Xe-O bonds and two lone-pair maxima forming an octahedron, consistent with the geometry proposed by recent experiment 146. Just as in XeO, there are no peaks visible in the ELF isosurface along the Xe-O bonds (Fig. 5.2b). Above 198 GPa XeO transforms into the XeO
transforms into the XeO -Cmcm structure. This non-trivial structure can be represented as having parallel zigzag Xe chains (Xe-Xe distances are 2.62 ), with each Xe atom having two neighboring oxygen atoms in the form reminiscent of bent (the Xe-O distance is 1.95 , O-Xe-O angle is 160.7
-Cmcm structure. This non-trivial structure can be represented as having parallel zigzag Xe chains (Xe-Xe distances are 2.62 ), with each Xe atom having two neighboring oxygen atoms in the form reminiscent of bent (the Xe-O distance is 1.95 , O-Xe-O angle is 160.7 ) XeO
) XeO molecules.
molecules.
XeO becomes stable at 114 GPa. Its structure has space group is P4
becomes stable at 114 GPa. Its structure has space group is P4 /mnm and 16 atoms in the unit cell. It is stable towards decomposition into Xe and O
/mnm and 16 atoms in the unit cell. It is stable towards decomposition into Xe and O as well as into XeO or XeO
as well as into XeO or XeO and O
and O . As shown in Supplementary Materials, P4
. As shown in Supplementary Materials, P4 /mnm phase is composed of two sublattices: square XeO
/mnm phase is composed of two sublattices: square XeO chains again, suggesting the Xe
chains again, suggesting the Xe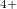 state and chains made of linear O
state and chains made of linear O dumbbells. Above 145 GPa, the molecules in the linear -O
dumbbells. Above 145 GPa, the molecules in the linear -O -O
-O - chains are partly dissociated and we observe the -O
- chains are partly dissociated and we observe the -O -O- chain in the C2/c phase with 48 atoms per unit cell. Above 198 GPa, the structure transforms to a Pmmn phase with 8 atoms per unit cell (Supplementary Materials). In this remarkable structure, the oxygen atoms form anticuboctahedrea in which the Xe atom sits in the center (Fig. 5.2c). The ELF distribution around Xe atoms in the Pmmn phase is spherical around the xenon, which points at the Xe
-O- chain in the C2/c phase with 48 atoms per unit cell. Above 198 GPa, the structure transforms to a Pmmn phase with 8 atoms per unit cell (Supplementary Materials). In this remarkable structure, the oxygen atoms form anticuboctahedrea in which the Xe atom sits in the center (Fig. 5.2c). The ELF distribution around Xe atoms in the Pmmn phase is spherical around the xenon, which points at the Xe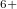 valence state with a spherically symmetric 5s
valence state with a spherically symmetric 5s valence shell. Again, we observe the tendency of increasing oxidation states under pressure.
valence shell. Again, we observe the tendency of increasing oxidation states under pressure.
Xenon fluorides are stable at ambient conditions, whereas xenon oxides become stable above 83 GPa. Xenon carbides are unstable up to 200 GPa at least 151. It appears that xenon forms compounds most readily with the most electronegative atoms, and that in turn suggests that ionicity is essential. This is somewhat counterintuitive, given that xenon atom has a very stable closed valence shell and its electronegativity is rather high. The electronegativity difference (1.4 for Xe-F, 0.8 for Xe-O and 0.56 for Xe-C) determines the degree of ionicity at ambient conditions. However, ionicity could be enhanced under pressure. Spontaneous ionization under pressure was recently found even in elemental boron 12.