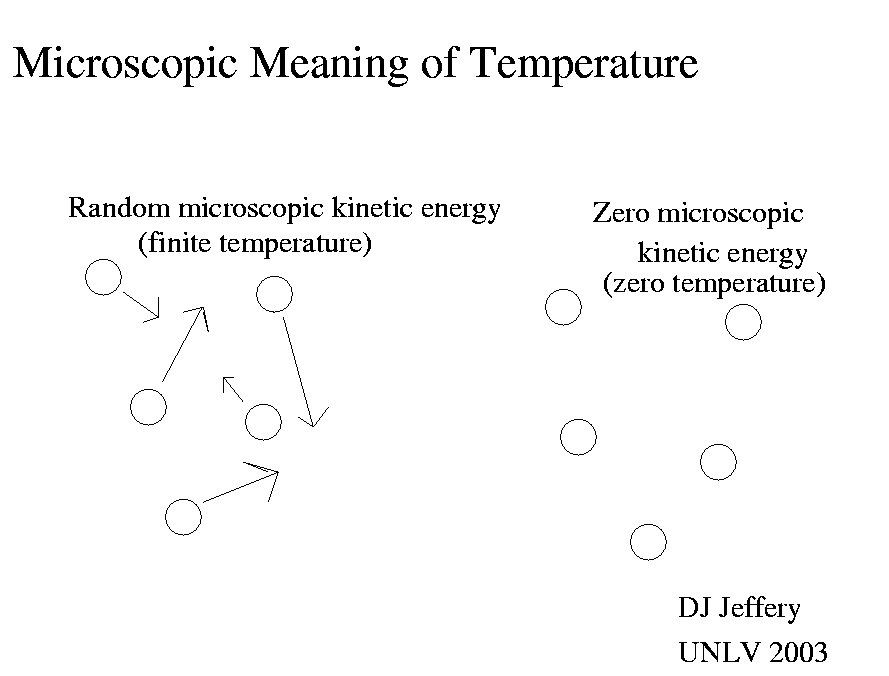
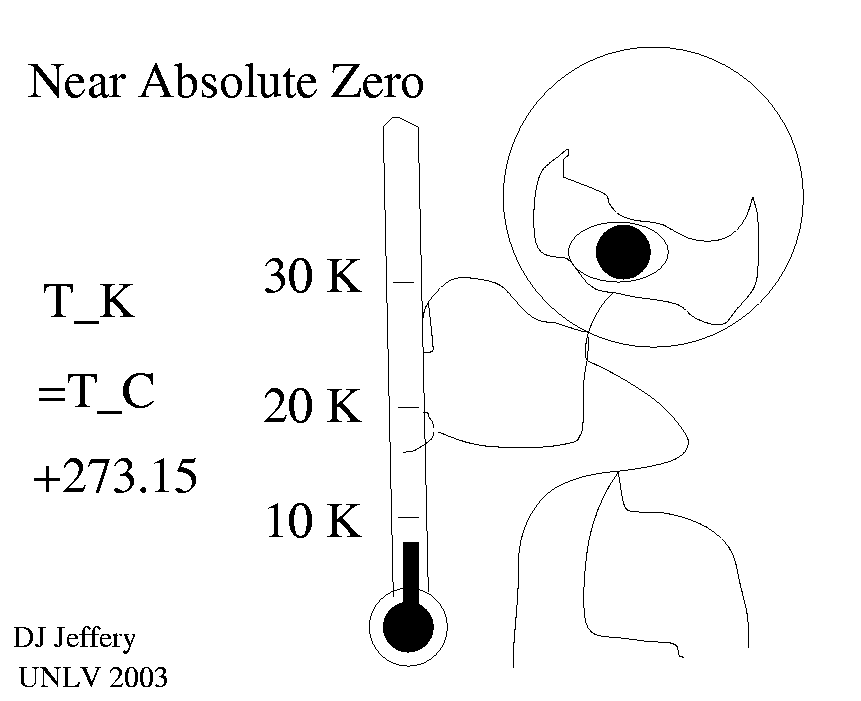 If you extract all the microscopic kinetic energy, then all microscopic motion ceases.
So if you want to set up a temperature scale, then choosing the condition
no microscopic kinetic energy to be zero temperature is very useful.
You never have to worry about negative temperatures and zero temperature has
a universal physical relevance: if something is at zero temperature, then it's
in a special physical condition that is the low temperature limit of all its behavior.
So in the Kelvin or absolute scale, zero temperature is defined classically
to be the condition of no microscopic kinetic energy.
The Kelvin scale was named in honor of
Lord Kelvin (William Thompson) (1824--1907) who had
a lot to with setting it up one supposes.
If you extract all the microscopic kinetic energy, then all microscopic motion ceases.
So if you want to set up a temperature scale, then choosing the condition
no microscopic kinetic energy to be zero temperature is very useful.
You never have to worry about negative temperatures and zero temperature has
a universal physical relevance: if something is at zero temperature, then it's
in a special physical condition that is the low temperature limit of all its behavior.
So in the Kelvin or absolute scale, zero temperature is defined classically
to be the condition of no microscopic kinetic energy.
The Kelvin scale was named in honor of
Lord Kelvin (William Thompson) (1824--1907) who had
a lot to with setting it up one supposes.
1 K = 1 degree C ,
where note the word degree or symbol degree is not used with the Kelvin scale. The relation between the Kelvin and Celsius scales is
T_K =T_C + 273.15 ,
which implies that -273.15 degrees C is absolute zero. Recall 0 degrees C is the freezing point of water and 100 degrees C is the boiling point of water. The relation of the Celsius scale to the Fahrenheit is
T_F =1.8*T_C + 32 .
 of temperature. Well macroscopically temperature is a measure of the thermal
equilibrium condition of a body.
If two bodies have the same temperature and you put them in thermal contact
(i.e., allow heat energy to flow between them), then no heat will in fact flow
spontaneously between them and the bodies do not change
(e.g., expand, contract, change phase, or experience pain).
Now if the bodies have a temperature difference, there will be a heat flow
and the bodies will change somehow: the larger the temperature difference, the
larger the heat flow and change.
In statistical mechanics, the microscopic and macroscopic
meanings of temperature are shown to be consistent.
of temperature. Well macroscopically temperature is a measure of the thermal
equilibrium condition of a body.
If two bodies have the same temperature and you put them in thermal contact
(i.e., allow heat energy to flow between them), then no heat will in fact flow
spontaneously between them and the bodies do not change
(e.g., expand, contract, change phase, or experience pain).
Now if the bodies have a temperature difference, there will be a heat flow
and the bodies will change somehow: the larger the temperature difference, the
larger the heat flow and change.
In statistical mechanics, the microscopic and macroscopic
meanings of temperature are shown to be consistent.
Table of Notable Temperatures
Notable Temperature Kelvin (K) Celsius (oC) Fahrenheit (oF)
absolute zero 0 -273.15 not worth knowing coincidence 233.15 -40 -40 water freezing 273.15 0 32 human warmish 300 26.85 80.33 water boiling 373.15 100 212 pure iron melting 1808 1535 who cares pure iron boiling 3023 2750 " Sun surface 5800 who cares " Sun center 15*10**6 " "
Sources: HRW-429, Se-147, and for iron EnvironmentalChemistry.com